Search Your Topic
Monosaccharides- Ring structures and Mutarotation
Representation of the ring structure of monosaccharides
The ring structures of monosaccharides can be represented as follows :
1) Fischer projection
The acyclic structure of a sugar is commonly shown by using a Fischer projection. A Fischer projection is sometimes used to illustrate the cyclic hemiacetal form of sugar. The presence of an aldehyde group and the hydroxyl groups in an aldose make it possible for these compounds to undergo intramolecular reactions to form cyclic hemiacetal. These cyclic hemiacetals are often more stable than is the open-chain form of the sugar. The ketoses (keto group containing sugars) form hemiketal rings

Figure-1- In Fischer projection, α anomer has the orientation of OH towards the right side, whereas, in beta anomer, it is towards the left side.
Rules for drawing Haworth projections
2) Haworth projection
Either a six or 5-membered ring is drawn including oxygen as one atom.
Most aldohexoses are six-membered resembling Pyran- an organic compound having a 6 membered ring (Figure-2).
Aldotetroses, aldopentoses, ketohexoses are 5-membered resembling Furan- A five-membered organic compound (Figure-2)
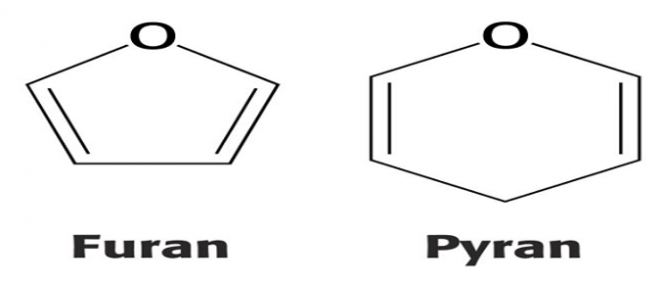
Figure-2- Structure of Furan and Pyran.
Numbering the rings
The numbering is done clockwise starting next to the oxygen (Figure-3)

Figure-3- Numbering the rings
Pyranose and Furanose forms of Glucose (Figure-4)

Figure-4- Glucopyranose and Glucofuranose forms
In Haworth configuration all groups to the right of carbon backbone in Fischer projection are oriented down (down -right) while all groups to the left of carbon backbone are oriented up, except those around C5, the reverse orientation occurs (Figure-5).
Alpha and beta anomers (Figure-5)

Figure-5- When drawn in the Haworth projection, the α configuration places the hydroxyl downward, while the β is the reverse.
Mutarotation
Carbohydrates can change spontaneously between α and β configurations through intermediate open-chain formation, this leads to a process known as Mutarotation. There is a gradual change in the optical rotation of the solution. The initial optical rotation is changed to a constant optical rotation characteristic of that sugar.
This can be explained in reference to two experiments
1) When D Glucose is crystallized at room temperature and a fresh solution is prepared, its specific rotation of polarized light is +112ο, but after 12-18 hours it changes to +52.5 ο
2) If the initial crystallization takes place at 98 ο and then solubilized, the specific rotation is found to be +19 ο, which also changes to +52.5 ο within a few hours.
This change in rotation with time is called Mutarotation.
Explanation
At room temperature, the alpha form predominates and the specific rotation is+112ο, there is transient ring-opening and change in configuration. In the second condition when the crystallization takes place at 98 ο, the Beta form predominates and the specific rotation is+19 ο. Both undergo Mutarotation and at equilibrium one-third molecules are α type and two-third are β variety to get the specific rotation of+52.5 ο (Figure-6).

Figure-6- Mutarotation of Glucose
The constant specific rotation is due to the resultant net optical activity of both alpha and beta anomers.
