Search Your Topic
Fatty acid synthesis- lecture 3 (fatty acid synthase complex)
Fatty acid synthase complex is the second most important enzyme complex in the pathway of de novo fatty acid biosynthesis. In mammals, the fatty acid synthase complex is a dimer comprising two identical monomers, each containing all seven enzyme activities of fatty acid synthase on one polypeptide chain. Each chain is folded into three domains joined by flexible regions (figure-1).
- Domain 1, condensing unit- the substrate entry and condensation unit, contains acetyltransferase, malonyl transferase, and β-ketoacyl synthase (condensing enzyme).
- Domain 2, the reduction unit, contains the acyl carrier protein, β-ketoacyl reductase, dehydratase, and enoyl reductase.
- Domain 3, the palmitate release unit, contains the Thioesterase.
Thus, seven different catalytic sites are present on a single polypeptide chain (figure 1&2).
In bacteria and plants, the individual enzymes of the fatty acid synthase system are separate, and the acyl radicals are found in combination with a protein called the acyl carrier protein (ACP). However, in yeast, mammals, and birds, the synthase system is a multienzyme polypeptide complex that incorporates ACP, which takes over the role of CoA. It contains vitamin pantothenic acid in the form of 4′-phosphopantetheine (figure-1&2).
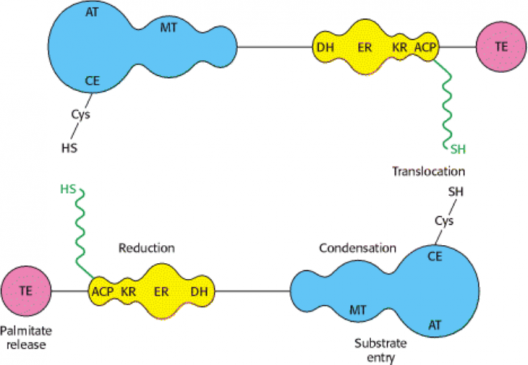
Figure-1-Schematic representation of Fatty Acid Synthase complex.-Each of the identical chains in the dimer contains three domains. Domain 1 (blue) contains acetyltransferase(AT), malonyl transferase (MT), and condensing enzyme (CE). Domain 2 (yellow) contains acyl carrier protein (ACP), b-ketoacyl reductase (KR), dehydratase (DH), and enoyl reductase (ER). Domain 3 (red) contains thioesterase (TE). The flexible phosphopantetheine group (green) carries the fatty acyl chain from one catalytic site on a chain to another, as well as between chains in the dimer.
Biological Advantage of having Multienzyme complex-
- An advantage of this arrangement is that the synthetic activity of different enzymes is coordinated since it is encoded by a single gene.
- A multienzyme complex consisting of covalently joined enzymes is more stable than one formed by noncovalent attractions.
- Furthermore, intermediates can be efficiently handed from one active site to another without leaving the assembly.

Figure-2- Fatty acid synthase multienzyme complex- The —SH of the 4′-phosphopantetheine of one monomer is in close proximity to the —SH of the cysteine residue of the ketoacyl synthase of the other monomer, suggesting a “head-to-tail” arrangement of the two monomers. Though each monomer contains all the partial activities of the reaction sequence, the actual functional unit consists of one-half of one monomer interacting with the complementary half of the other. Thus, two acyl chains are produced simultaneously.
