Search Your Topic
Chromatography
The word Chromatography is derived from two Greek words –Chroma means – color and graphein to write. Chromatography is the collective term for a family of laboratory techniques for the separation of mixtures.
Chromatography, literally “color writing”, was used—and named— in the first decade of the 20th century, primarily for the separation of plant pigments such as chlorophyll.
Chromatography is a misnomer since it is no longer limited to the separation of the colored substances.
Principle- Chromatography is based on the principle of the partition of the solute between two phases/solvents. It usually consists of a mobile phase and a stationary phase. The mobile phase usually refers to the mixture of the substances to be separated dissolved in a liquid or a gas. The stationary phase is a porous solid matrix through which the sample contained in the mobile phase percolates. The interaction between the mobile and the stationary phases results in the separation of the compounds from the mixture. These interactions include the physicochemical principles such as the adsorption, ion- exchange, molecular sieving and affinity
Classification-There are two ways to classify the methodology of chromatography-
A) Based on nature of interactions between the sample components and the stationary phase, whereby the components are retarded to different degrees in their migration with the mobile phase and are consequently separated from each other- Following are the different types of chromatographic procedures-
1) Partition chromatography
2) Adsorption chromatography
3) Ion – Exchange chromatography
4) Gel filtration chromatography
5) Affinity chromatography
6) High- performance liquid chromatography
B) Base on the nature of the stationary phase or mobile phase
It is of two types
1) Planar- It may be Paper or Thin layer
2) Column- it may be Gas or Liquid
Planar chromatography–
- Planar chromatography is a separation technique in which the stationary phase is present as or on a plane.
- The plane can be a paper, serving as such or impregnated by a substance as the stationary bed (paper chromatography) or a layer of solid particles spread on a support such as a glass plate (thin layer chromatography).
- Different compounds in the sample mixture travel different distances according to how strongly they interact with the stationary phase as compared to the mobile phase.
- The specific Retardation factor (Rf) of each chemical can be used to aid in the identification of an unknown substance.
Column Chromatography
- Column chromatography is a separation technique in which the stationary bed is within a tube.
- The particles of the solid stationary phase or the support coated with a liquid stationary phase may fill the whole inside volume of the tube (packed column) or be concentrated on or along the inside tube wall leaving an open, unrestricted path for the mobile phase in the middle part of the tube (open tubular column).
- Differences in rates of movement through the medium are calculated to different retention times of the sample.
1. Partition Chromatography
This is more commonly used for the separation of a mixture of amino acids and peptides. The molecules of a mixture get partitioned between the stationary and the mobile phase depending on the relative affinity of each one of the phases.
a) Paper chromatography- Paper chromatography is an analytical technique for separating and identifying mixtures that are or can be colored especially pigments. This method has been largely replaced by thin-layer chromatography, however, it is still a powerful technique. It is a liquid-liquid partition chromatography. The stationary phase is water held on a solid support of filter paper (cellulose). The mobile phase is a mixture of immiscible solvents which are mixtures of water, a nonpolar solvent and an acid or base e.g. Butanol, acetic acid water or phenol-water-ammonia.
Technique(Figure-1)
- A small concentrated spot of solution that contains the sample of the solute is applied to a strip of chromatography paper about two centimeters away from the base of the plate.
- This sample is absorbed onto the paper and may form interactions with it.
- Any substance that reacts or bonds with the paper cannot be measured using this technique.
- The paper is then dipped into a suitable solvent, such as ethanol or water, taking care that the spot is above the surface of the solvent, and placed in a sealed container.
- The solvent moves up the paper by capillary action, which occurs as a result of the attraction of the solvent molecules to the paper. (Figure-1)
- As the solvent rises through the paper it meets and dissolves the sample mixture, which will then travel up the paper with the solvent-solute sample.
- Different compounds in the sample mixture travel at different rates due to differences in solubility in the solvent, and due to differences in their attraction to the fibers in the paper.
- Paper chromatography takes anywhere from several minutes to several hours.
- In some cases, paper chromatography does not separate pigments completely; this occurs when two substances appear to have the same values in a particular solvent.
- In these cases, two-way chromatography is used to separate the multiple-pigment spots.

Figure-1 showing paper chromatography(Ascending)
Ascending Chromatography
In this method, the solvent is in the pool at the bottom of the vessel in which the paper is supported. It rises up the paper by capillary action against the force of gravity (Figure-1).
Descending Chromatography
In this method, the solvent is kept in a trough at the top of the chamber and is allowed to flow down the paper. The liquid moves down by capillary action as well as by the gravitational force. In this case, the flow is more rapid as compared to the ascending method. Because of this rapid speed, the chromatography is completed in a comparatively shorter time.

Figure-2- showing descending paper chromatography. The developing solvent is placed in a trough at the top which is usually made up of inert material. The paper is then suspended in the solvent. Substances that cannot be separated by the ascending method, can be separated by the above descending method.
Analysis– After development, the spots corresponding to different compounds may be located by their color, ultraviolet light, ninhydrin or by treatment with iodine vapors. The paper remaining after the experiment is known as the Chromatogram.
Composition of Filter Paper
The original work in paper chromatography was carried on the Whatman no.1 filter paper. These days paper for chromatography is made from cotton cellulose.
Rƒ value
The components which have been separated differ in their retention factor i.e Ratio of distance traveled from the spot or origin by the solute component to that of the distance traveled from the spot or origin by the solvent. Retention Factor can never be greater than one.
Rf, – Distance traveled by sample/ distance traveled by the solvent.
The final chromatogram can be compared with other known mixture chromatograms to identify sample mixes using the Rf value in an experiment. The retention values found can be compared to known values, and from that conclusions can be drawn.
Rƒ values are usually expressed as a fraction of two decimal places.
If Rƒ value of a solution is zero, the solute remains in the stationary phase and thus it is immobile. If Rƒ value = 1 then the solute has no affinity for the stationary phase and travels with the solvent front.
Two-dimensional chromatography– Sometimes, it is difficult to separate a complex mixture of substances by a single run with one solvent system. In such a case, a second run is carried out by a different solvent system, in a direction perpendicular to the first run. This is referred to as- two-dimensional chromatography which enhances the separation of a mixture into individual components. (Figure-3)

Figure-3- showing two-dimensional paper chromatography
Importance-
1) Paper chromatography is a very easy, simple, rapid and highly efficient method of separation.
2) It can be applied even in microgram quantities of the sample.
3)It can also be used for the separation of a wide variety of materials like amino acids, oligopeptides, sugars, oligosaccharides, glycosides, purines and pyrimidines, steroids, vitamins and some alkaloids like penicillin, tetracycline, and streptomycin.
4) It is not preferred for separating proteins because they are not soluble in many of the solvent systems and are also denatured by them. Paper chromatography is inferior to thin layer chromatography in resolving power.
b) Thin-layer chromatography (TLC) is a chromatographic technique used to separate mixtures. It involves a stationary phase consisting of a thin layer of adsorbent material, usually silica gel, aluminum oxide, or cellulose immobilized onto a flat, inert carrier sheet. A liquid phase consisting of the solution to be separated is then dissolved in an appropriate solvent and is drawn up the plate via capillary action, separating the experimental solution based on the polarity of the components of the compound in question.
Importance of TLC– Its wide range of uses include
- determination of the pigments a plant contains
- detection of pesticides or insecticides in food
- identifying compounds present in a given substance
- monitoring organic reaction
Technique
The process is similar to paper chromatography with the advantage of faster runs, better separations, and the choice between different stationary phases. Because of its simplicity and speed, TLC is often used for monitoring chemical reactions and for the qualitative analysis of reaction products.
A small spot of the solution containing the sample is applied to a plate, about one centimeter from the base. The plate is then dipped into a suitable solvent, such as hexane or ethyl acetate, and placed in a sealed container. The solvent moves up the plate by capillary action and meets the sample mixture, which is dissolved and is carried up the plate by the solvent. Different compounds in the sample mixture travel at different rates due to the differences in their attraction to the stationary phase, and because of differences in solubility in the solvent.(Figure-4).
The separation of compounds is based on the competition of the solute and the mobile phase for binding places on the stationary phase. For instance, if normal phase silica gel is used as the stationary phase it can be considered polar. Given two compounds which differ in polarity, the most polar compound has a stronger interaction with the silica and is, therefore, more capable to dispel the mobile phase from the binding places. Consequently, the less polar compound moves higher up the plate (resulting in a higher Rf value).

Figure-4- showing the mechanism of thin-layer chromatography
Analysis
As the chemicals being separated may be colorless, several methods exist to visualize the spots:
- Often a small amount of a fluorescent compound, usually manganese-activated zinc silicate, is added to the adsorbent that allows the visualization of spots under a blacklight (UV254)..
- Iodine vapors are a general unspecific color reagent
- Ninhydrin is used for amino acids and proteins
- Sulphuric acid is used for phospholipids
Once visible, the Rf value, or Retention factor, of each spot can be determined. These values depend on the solvent used, and the type of TLC plate, and are not physical constants. The Rf value for compounds (amino acids, peptides, sugars, fatty acids, phospholipids, etc.) for commonly used solvent systems has been calculated and available for comparison.TLC can also be used for two-dimensional chromatography using the same plate with two solvent systems, as in the case of paper chromatography.
Advantages of TLC over paper chromatography-
1) In the case of paper chromatography, it takes 14-16 hrs for the separation of the components, but in TLC, It takes only 3-4 hrs.
2) TLC has the advantage that corrosive reagents like sulphuric acid can also be used which poses a limitation for the paper chromatography.
3) It is easier to separate and visualize the components by this method.
4) It has the capacity to analyze multiple samples in a single run.
5) It is relatively a low cost.
2)Adsorption chromatography
- In this technique, the separation is based on differences in adsorption at the surface of the solid stationary medium.
- The adsorbents such as silica gel, charcoal powder, and calcium hydroxyapatite are packed into a column in a glass tube.
- This serves as the stationary phase. The sample mixture in a solvent is loaded on this column.
- The individual components get differentially adsorbed on to the adsorbent (Figure-5).
- The elution is carried out by a buffer system (mobile phase).
- The most weakly held fraction moves fastest, followed by others, according to the order of tightness in adsorption.
- The individual compounds come out of the column at different rates which may be separately collected and identified

Figure-5- showing adsorption chromatography
3)Ion – Exchange chromatography
- Ion-exchange chromatography (or ion chromatography) is a process that allows the separation of ions and polar molecules based on the charge properties of the molecules (Figure-6).
- It can be used for almost any kind of charged molecule including large proteins, small nucleotides, and amino acids.
- The solution to be injected is usually called a sample, and the individually separated components are called analytes. It is often used in protein purification, water analysis, and quality control.
- Ion exchange chromatography retains analyte molecules based on coulombic (ionic) interactions.
- The stationary phase surface displays ionic functional groups that interact with analyte ions of the opposite charge. This type of chromatography is further subdivided into cation exchange chromatography and anion exchange chromatography. The ionic compound consisting of the cationic species and the anionic species can be retained by the stationary phase.

Figure-6 -showing ion exchange chromatography
Cation exchange chromatography retains positively charged cations because the stationary phase displays a negatively charged functional group.
Cation Exchange resins- Polystyrene sulfonate resins, CM- Sephadex gel, CM – cellulose, These bear acidic groups and immobilize cations from adjacent solutions.
Anion exchange chromatography retains anions using a positively charged functional group.
Anion exchangers- DEAE cellulose, Trimethyl amino polystyrene, DEAE- Sephadex. All these bear basic groups ionizing into fixed positions and immobilize anions from neighboring solutions.
The ionic groups in cation exchange resins are sulphonic and carboxyl groups, while the anion exchange resins have quaternary nitrogen.
4) Gel filtration chromatography or Molecular Sieve chromatography-
- This is extremely useful in separating ribosomes, viruses, nucleic acids, and proteins depending on their particle sizes and shapes.
- In Gel filtration chromatography, the separation of the particles is based on their size, shape and molecular weight.
- This technique is also referred to as molecular exclusion chromatography.
- The apparatus consists of a column packed with sponge-like gel beads( usually cross-linked polysaccharides) containing pores.
- The gels serve as molecular sieves for the separation of smaller and bigger molecules
- The solution mixture containing molecules of different sizes( say protein) is applied to the column and eluted with a buffer. (Figure-7).
- The larger molecules cannot pass through the pores of a gel and therefore move faster.
- On the other hand, the smaller molecules enter the gel beads and are left behind which come out slowly.
- By selecting the gel beads of different porosity, the molecules can be separated.
- The commercially available gels include (G-10, G-25,G-100), Bio gel(P-10,P-30,P-100) and Sepharose(6B,4B,2B).
- The gel filtration chromatography can be used for an approximate determination of molecular weights.
- This is done by using a calibrated column with substances of known molecular weights.

Figure-7- showing the mechanism of molecular sieve chromatography.
5) Affinity chromatography
- The principle of affinity chromatography is based on the property of specific and non-covalent binding of proteins to other molecules, referred to as substrates or cofactors.
- The technique involves the use of ligands covalently attached to an inert and porous matrix in a column.
- The immobilized ligands act as molecular hooks to selectively pick up the desired protein while the remaining proteins pass through the column.
- The desired protein captured by the ligand can be eluted by using free ligand molecules.
- Alternatively, some reagents that can break protein-ligand interactions can also be employed for the separation (Figure-8)
- Affinity chromatography is useful for the purification of enzymes, vitamins, nucleic acids, drugs, hormone receptors, antibodies, etc.
- For example, NAD is used to purify dehydrogenases. By using antibodies, antigens can be easily separated.
- Conversely, antibodies can be purified by passing through a column containing the antigen.
- It is also widely used for the estimation of glycated Hb. Normal Hb does not bind and comes out first, while glycated Hb binds with the Boronic acid used as a ligand. Sorbitol is then added to elute the glycated Hb which can be quantitated then.
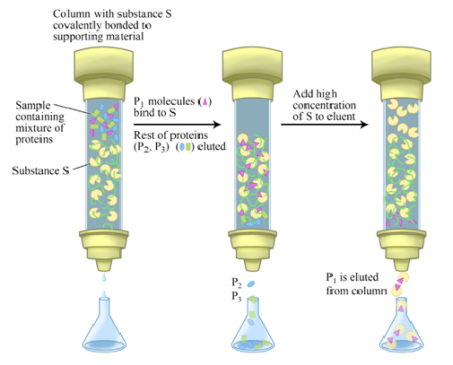
Figure-8- showing the mechanism of Affinity chromatography
6) High-performance liquid chromatography
- In general chromatographic techniques are slow and time-consuming.
- The separation can be greatly improved by applying high pressure in the range of 5000-10,000 pounds per square inch, hence this technique is also referred to as high-pressure liquid chromatography.
- HPLC requires the use of non-compressible resin materials and strong metal columns.
- The eluents of the columns are detected by methods such as UV absorption and fluorescence.
- It can be applied in the form of partition, adsorption, ion exchange or molecular sieve chromatography.
- The stationary phase consists of an immobilized thin layer of a liquid on the micro-glass or plastic beads, tightly packed into a narrow column. (Figure-9)
- The mobile phase consists of a buffered solvent system that is passed under high pressure through the column for eluting the solutes of the sample.

Figure-9- showing the apparatus for high-performance liquid chromatography
Due to rapidity in action, it is used for assaying amino acids, peptides, proteins, carbohydrates, lipids, nucleic acids and related compounds, vitamins, hormones, metabolites and drugs such as antiarrhythmics, antibiotics, antiepileptics, analgesics, bronchial smooth muscle relaxants, and anti-depressants.
6) Gas-liquid chromatography-
- This is the method of choice for the separation of volatile substances or volatile derivatives of certain in volatile substances.
- In GLC. the stationary phase is an inert solid material(diatomaceous earth or powdered firebrick), impregnated with a non-volatile liquid(silicon or polyethylene glycol).
- This is packed in a narrow column and maintained at high temperatures (around 200 degrees C).
- A mixture of a volatile material is injected into the column along with the mobile phase, which is an inert gas (argon, helium or nitrogen).
- The separation of the volatile material is based on the partition of the components between the mobile phase (gas) and stationary phase( liquid), hence the name gas-liquid chromatography. (Figure-10)
- The separated compounds can be identified and quantitated by a detector. Gas-liquid chromatography is sensitive, rapid and reliable.
- It is frequently used for the quantitative estimations of biological materials such as lipids, drugs, and vitamins.

Figure-10- showing apparatus for gas-liquid chromatography.
