Search Your Topic
Case study- Porphyria
A 30-year-old woman had severe abdominal pain, nausea, vomiting and diarrhea. Evaluations including upper and lower endoscopies did not establish any intestinal infection. She gradually improved and was discharged after 2 weeks. 2 years later she was admitted to a psychiatric unit with acute mental changes and hallucinations, she had to be transferred to the emergency department due to abdominal pain, a grand mal seizure, and hyponatremia.
Her pulse was 120 and BP 174/114 mm Hg. She was disoriented but had no focal neurological signs. MRI showed subcortical abnormalities, and the spinal fluid was normal. After cholecystectomy for a distended gallbladder, she was discharged but she stayed with a family member in another city because her symptoms were worse and muscle weakness had developed. She was hospitalized and progressed to quadriparesis, respiratory failure, and aspiration pneumonia. Urinary porphobilinogen (PBG) was reported as 44 mg/24 hours (reference range 0-4).
What is the diagnosis and defect in this disease?
How is the diagnosis done and what is its prognosis?
What is the genetic basis of this disease?
Case Discussion
The patient is suffering from acute intermittent Porphyria as evident from the typical combination of abdominal pain, motor neuropathy, psychiatric symptoms and increased amounts of urinary Porphyrins and their precursors. Acute intermittent Porphyria (AIP) is a rare autosomal dominant metabolic disorder affecting the production of heme. It is characterized by a deficiency of enzyme porphobilinogen deaminase.
Basic concept
Haem synthesis
Heme is required for a variety of hemoproteins such as hemoglobin, myoglobin, respiratory Cytochromes, and the cytochrome P450 enzymes (CYPs). Hemoglobin synthesis in erythroid precursor cells accounts for approximately 85% of daily heme synthesis in humans. Hepatocytes account for most of the rest, primarily for synthesis of CYPs, which are especially abundant in the liver endoplasmic reticulum, and turn over more rapidly than many other hemoproteins, such as the mitochondrial respiratory cytochromes.
Heme biosynthesis involves eight enzymatic steps in the conversion of glycine and succinyl-CoA to heme (Fig.1). These eight enzymes are encoded by nine genes, as the first enzyme in the pathway, 5′-aminolevulinate synthase (ALA-synthase), has two genes that encode unique housekeeping (ALAS1) and erythroid-specific (ALAS2) isozymes. The first and last three enzymes in the pathway are located in the mitochondrion, whereas the other four are in the cytosol. As shown in Fig.1, pathway intermediates are the porphyrin precursors, ALA and PBG, and porphyrins (mostly in their reduced forms, known as porphyrinogens). At least in humans, these intermediates do not accumulate in significant amounts under normal conditions or have important physiologic functions.

Figure-1- showing the steps of Haem synthesis
Steps of Haem synthesis
1) The first and rate-controlling step is the condensation of glycine and succinyl–coenzyme A (CoA) to form δ-aminolevulinic acid (ALA). The enzyme, ALA-synthase is activated by Pyridoxal phosphate. In the liver, this rate-limiting enzyme can be induced by a variety of drugs, steroids, and other chemicals. Defects in the erythroid gene, cause X-linked Sideroblastic anemia (XLSA).
2) The ALA formed is transported into the cytoplasm, where the second enzyme, ALA dehydratase (also known as porphobilinogen synthase), condenses two molecules of ALA to form the monopyrrole porphobilinogen.
3) The third enzyme, porphobilinogen deaminase (also known as hydroxymethylbilane synthase), forms a linear tetrapyrrole, hydroxymethylbilane, which is normally rapidly converted, mainly to the cyclic intermediate Uroporphyrinogen III, by the enzyme Uroporphyrinogen III synthase (also known as Uroporphyrinogen co-synthase). When Uroporphyrinogen III synthase is deficient, as in congenital erythropoietin Porphyria (Guenther’s disease), hydroxymethylbilane rapidly undergoes nonenzymatic ring closure to form Uroporphyrinogen I.
4) The enzyme Uroporphyrinogen decarboxylase carries out the stepwise decarboxylation of Uroporphyrinogen I or III to form intermediates with 7-, 6-, 5-, and 4-carboxyl groups. Coproporphyrinogen is the common name for the 4-carboxyl–containing intermediate.
5) Coproporphyrinogen III is transported back into mitochondria, where the enzyme Coproporphyrinogen III oxidase carries out the stepwise oxidative decarboxylation of two of the remaining propionate beta side chains, at positions 2 and 4 (on rings A and B, respectively), to vinyl groups, forming protoporphyrinogen IX.
6) Next, the enzyme protoporphyrinogen oxidase carries out the oxidation of protoporphyrinogen IX to form protoporphyrin IX, after which the enzyme Ferrochelatase (also called heme synthase) inserts ferrous iron into the protoporphyrin IX macrocycle to form the end product heme. (See figure-1)
Regulation of Heme Biosynthesis
Regulation of heme synthesis differs in the two major heme-forming tissues, the liver, and erythron. In the liver, “free” heme regulates the synthesis and mitochondrial translocation of the housekeeping form of ALA-synthase. Heme represses the synthesis of the ALA-synthase mRNA and interferes with the transport of the enzyme from the cytosol into mitochondria. Hepatic ALA-synthase is increased by many of the same chemicals that induce the cytochrome P450 enzymes in the endoplasmic reticulum of the liver. Because most of the heme in the liver is used for the synthesis of cytochrome P450 enzymes, hepatic ALA-synthase and the cytochrome P450s are regulated in a coordinated fashion, and many drugs that induce hepatic ALA-synthase also induce CYPs. The other hepatic heme biosynthetic enzymes are presumably expressed at constant levels, although their relative activities and kinetic properties differ. For example, normal individuals have high activities of ALA-dehydratase but low activities of HMB-synthase, the latter being the second rate-limiting step in the pathway.
In the erythron, novel regulatory mechanisms allow for the production of the very large amounts of heme needed for hemoglobin synthesis. The response to stimuli for hemoglobin synthesis occurs during cell differentiation, leading to an increase in cell number. The erythroid-specific ALA-synthase is expressed at higher levels than the housekeeping enzyme, and erythroid-specific control mechanisms regulate other pathway enzymes as well as iron transport into erythroid cells.
PORPHYRIAS
The Porphyrias are a group of rare metabolic disorders arising from reduced activity of any of the enzymes in the heme biosynthetic pathway. The disorders may be either acquired or inherited through a genetic defect in a gene encoding these enzymes. These deficiencies disrupt normal heme production and produce symptoms when increased heme is required. Porphyrin precursors, overproduced in response to synthetic pathway blockages, accumulate in the body and cause diverse pathologic changes thereby becoming the basis for diagnostic tests.
The diagnosis of acute porphyrias can be confirmed by repeating the quantitation of urinary porphyrin during an acute episode and finding elevated levels (2–5 times of normal) of porphobilinogen.
Incidence
The prevalence of this condition is unknown but probably ranges from 1 in 500 to 50,000 worldwide. Certain types of porphyrias are more common in specific populations, such as whites in South Africa and Scandinavians.
Classification
The Porphyrias can be classified as either hepatic or erythropoietic, depending on whether the heme biosynthetic intermediates that accumulate arise initially from the liver or developing erythrocytes or as acute or cutaneous, based on their clinical severity.
Inheritance
Most of the Porphyrias are inherited. Inheritance patterns depend on the type of porphyria. Some forms of the condition are inherited in an autosomal dominant pattern, which means one copy of the altered gene is sufficient to cause the disorder. Other Porphyrias are inherited in an autosomal recessive pattern, which means two copies of the gene must be altered for a person to be affected by the condition.
Typically, patients with the autosomal dominant varieties present initially in adulthood, and those with homozygous variants present in early childhood. Symptomatic porphyria is thought to be more common in female than male patients with a female-male ratio of 5 to 1.
A) The Hepatic Porphyrias
1) ALA-Dehydratase Deficient Porphyria (ADP)
ADP is a rare autosomal recessive acute hepatic porphyria caused by a severe deficiency of ALA-dehydratase activity. The clinical presentation depends on the amount of residual ALA-dehydratase activity. Symptoms resemble those of AIP (Acute intermittent Porphyria) including abdominal pain and neuropathy. An infant with more severe disease manifest failure to thrive beginning at birth. Diagnosis is confirmed by significantly elevated levels of plasma and urinary ALA, urinary coproporphyrin III and ALAD activity in erythrocytes <10% of normal.
The treatment of ADP acute attacks is similar to that of AIP.
2) Acute Intermittent Porphyria (AIP)
Acute intermittent porphyria is inherited as an autosomal dominant, though it remains clinically silent in most patients who carry the trait. The clinical illness usually develops in women. Symptoms begin in the teens or 20s, but onset can begin after menopause in rare cases. The disorder is caused by a partial deficiency of porphobilinogen deaminase activity, leading to increased excretion of aminolevulinic acid and porphobilinogen in the urine. The diagnosis may be elusive if not specifically considered. The characteristic abdominal pain may be due to abnormalities in autonomic innervations in the gut. In contrast to other forms of porphyria, cutaneous photosensitivity is absent in acute intermittent porphyria. Attacks are precipitated by numerous factors, including drugs and intercurrent infections. Hyponatremia may be seen, due in part to inappropriate release of antidiuretic hormone, though the gastrointestinal loss of sodium in some patients may be a contributing factor.
Clinical Findings
Symptoms and Signs
Patients show intermittent abdominal pain of varying severity, and in some instances, it may so simulate an acute abdomen as to lead to exploratory laparotomy. Complete recovery between attacks is usual. Any part of the nervous system may be involved, with evidence for autonomic and peripheral neuropathy. Peripheral neuropathy may be symmetric or asymmetric and mild or profound; in the latter instance, it can even lead to quadriplegia with respiratory paralysis. Other central nervous system manifestations include seizures, psychosis, and abnormalities of the basal ganglia. Hyponatremia may further cause or exacerbate central nervous system manifestations.
Laboratory Findings
Often there is profound hyponatremia. The diagnosis can be confirmed by demonstrating an increased amount of porphobilinogen in the urine during an acute attack. Freshly voided urine is of normal color but may turn dark upon standing in light and air.
Prevention
Avoidance of factors known to precipitate attacks of acute intermittent porphyria—especially drugs can reduce morbidity. Starvation diets also cause attacks and so must be avoided.
Treatment
Treatment with a high-carbohydrate diet diminishes the number of attacks in some patients and is a reasonable empiric gesture considering its benignity. Acute attacks may be life-threatening and require prompt diagnosis, withdrawal of the inciting agent (if possible), and treatment with analgesics and intravenous glucose and hematin. Electrolyte balance requires close attention. Liver transplantation may provide an option for patients with disease poorly controlled by medical therapy.
3) Porphyria Cutanea Tarda
PCT, the most common of the Porphyrias, can be either sporadic (type 1) or familial (types 2 and 3) and can also develop after exposure to halogenated aromatic hydrocarbons. Hepatic URO-decarboxylase is deficient in all types of PCT, and for clinical symptoms to manifest, this enzyme deficiency must be substantial (~20% of normal activity or less).
Clinical Features
Blistering skin lesions that appear most commonly on the backs of the hands are the major clinical feature.These rupture and crust over, leaving areas of atrophy and scarring. Lesions may also occur on the forearms, face, legs, and feet. Occasionally, the skin over sun-exposed areas becomes severely thickened, with scarring and calcification that resembles systemic sclerosis. Neurologic features are absent.
A number of susceptibility factors can be recognized clinically and can affect management. These include hepatitis C, HIV, excess alcohol, elevated iron levels, and estrogens. Excess alcohol is a long-recognized contributor, as is estrogen use in women. Multiple susceptibility factors that appear to act synergistically can be identified in the individual patient with PCT. Patients with PCT characteristically have chronic liver disease and sometimes cirrhosis and are at risk for hepatocellular carcinoma.
Diagnosis
Porphyrins are increased in the liver, plasma, urine, and stool. The urinary ALA level may be slightly increased, but the PBG level is normal. Urinary porphyrins consist mostly of uroporphyrin with lesser amounts of coproporphyrin. Plasma porphyrins are also increased.
Porphyria Cutanea Tarda: Treatment
Alcohol, estrogens, iron supplements, and, if possible, any drugs that may exacerbate the disease should be discontinued, but this step does not always lead to improvement. A complete response can almost always be achieved by the standard therapy, repeated phlebotomy, to reduce hepatic iron. A unit (450 mL) of blood can be removed every 1–2 weeks. The aim is to gradually reduce excess hepatic iron until the serum ferritin reaches the lower limits of normal. Because iron overload is not marked in most cases, remission may occur after only five or six phlebotomies; however, PCT patients with hemochromatosis may require more treatments to bring their iron levels down to the normal range.
An alternative when phlebotomy is contraindicated or poorly tolerated is a low-dose regimen of chloroquine or hydroxychloroquine, both of which complex with the excess porphyrins and promote their excretion. Hepatic imaging can diagnose or exclude complicating hepatocellular carcinoma. Treatment of PCT in patients with end-stage renal disease is facilitated by the administration of erythropoietin.
Sunscreen lotions and beta carotene are recommended to prevent skin damage caused by sunlight.
4) Hereditary Coproporphyria
HCP is an autosomal dominant hepatic porphyria that results from the half-normal activity of COPRO-oxidase. The disease presents with acute attacks, as in AIP. Cutaneous photosensitivity also may occur, but much less commonly than in VP. HCP patients may have acute attacks and cutaneous photosensitivity together or separately. HCP is less common than AIP and VP.
Clinical Features
HCP is influenced by the same factors that cause attacks in AIP. The disease is latent before puberty, and symptoms, which are virtually identical to those of AIP, are more common in women. HCP is generally less severe than AIP. Blistering skin lesions are identical to PCT and VP.
Diagnosis
COPRO III is markedly increased in the urine and feces in symptomatic disease and often persists, especially in feces, when there are no symptoms. Urinary ALA and PBG levels are increased (but less than in AIP) during acute attacks but may revert to normal more quickly than in AIP when symptoms resolve. Plasma porphyrins are usually normal or only slightly increased, but they may be higher in cases with skin lesions. The diagnosis of HCP is readily confirmed by increased fecal porphyrins consisting almost entirely of COPRO III, which distinguishes it from other porphyrias. An increase in the fecal COPRO III/COPRO I ratio is useful for detecting latent cases.
Although the diagnosis can be confirmed by measuring COPRO-oxidase activity, the assays for this mitochondrial enzyme are not widely available and require cells other than erythrocytes.
Hereditary Coproporphyria: Treatment
Neurologic symptoms are treated as in AIP . Phlebotomy and chloroquine are ineffective when cutaneous lesions are present.
5) Variegate Porphyria
VP is an autosomal dominant hepatic porphyria that results from the deficient activity of PROTO-oxidase, the seventh enzyme in the heme pathway, and can present with neurologic symptoms, photosensitivity, or both. VP is particularly common in South Africa, where 3 of every 1000 whites have the disorder.
Clinical Features
VP can present with skin photosensitivity, acute neurovisceral crises, or both. Acute attacks are identical to those in AIP and are precipitated by the same factors as AIP. Blistering skin manifestations are similar to those in PCT but are more difficult to treat and usually are of longer duration. Homozygous VP is associated with photosensitivity, neurologic symptoms, and developmental disturbances, including growth retardation, in infancy or childhood.
Diagnosis
Urinary ALA and PBG levels are increased during acute attacks but may return to normal more quickly than in AIP. Increases in fecal protoporphyrin and COPRO III and in urinary COPRO III are more persistent. Plasma porphyrin levels also are increased, particularly when there are cutaneous lesions. VP can be distinguished rapidly from all other porphyrias by examining the fluorescence emission spectrum of porphyrins in plasma at neutral pH since VP has a unique fluorescence peak at neutral pH.
Assays of PROTO-oxidase activity in cultured fibroblasts or lymphocytes are not widely available.
Variegate Porphyria: Treatment
Acute attacks are treated as in AIP, and hemin should be started early in most cases. Other than avoiding sun exposure, there are few effective measures for treating skin lesions. Beta-Carotene, phlebotomy, and chloroquine are not helpful.
B) The Erythropoietic Porphyrias
In the erythropoietic porphyrias, excess porphyrins from bone marrow erythrocyte precursors are transported via the plasma to the skin and lead to cutaneous photosensitivity.
1) X-Linked Sideroblastic Anemia
XLSA results from the deficient activity of the erythroid form of ALA-synthase and is associated with ineffective erythropoiesis, weakness, and pallor.
Clinical Features
Typically, males with XLSA develop refractory hemolytic anemia, pallor, and weakness during infancy. They have secondary hypersplenism, become iron overloaded, and can develop hemosiderosis. The severity depends on the level of residual erythroid ALA-synthase activity and on the responsiveness of the specific mutation to Pyridoxal 5´-phosphate supplementation.
Diagnosis
Peripheral blood smear reveals hypochromic, microcytic anemia with striking anisocytosis, poikilocytosis, and polychromasia; the leukocytes and platelets appear normal. Hemoglobin content is reduced, and the mean corpuscular volume and mean corpuscular hemoglobin concentration are decreased.
Bone marrow examination reveals hypercellularity with a left shift, megaloblastic erythropoiesis with an abnormal maturation. A variety of Prussian blue–staining sideroblasts are observed.
Levels of urinary porphyrin precursors and of both urinary and fecal porphyrins are normal. The level of erythroid ALA-synthase is decreased in bone marrow, but this enzyme is difficult to measure in the presence of the normal ALA-synthase housekeeping enzyme. Definitive diagnosis requires the demonstration of mutations in the erythroid ALAS gene.
X-Linked Sideroblastic Anemia: Treatment
The severe anemia may respond to pyridoxine supplementation. This cofactor is essential for ALA-synthase activity, and mutations in the pyridoxine binding site of the enzyme have been found in several responsive patients. Cofactor supplementation may make it possible to eliminate or reduce the frequency of transfusion. Unresponsive patients may be transfusion-dependent and require chelation therapy.
2) Congenital Erythropoietic Porphyria
CEP, also known as Günther disease, is an autosomal recessive disorder. It is due to the markedly deficient, but not absent, the activity of URO-synthase and the resultant accumulation of uroporphyrin I and coproporphyrin I isomers. CEP is associated with hemolytic anemia and cutaneous lesions.
Clinical Features
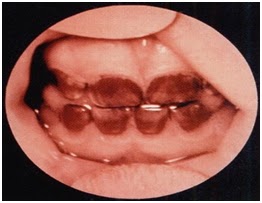
Severe cutaneous photosensitivity begins in early infancy. The skin over light-exposed areas is friable, and bullae and vesicles are prone to rupture and infection. Skin thickening, hypo- and hyperpigmentation, and hypertrichosis of the face and extremities are characteristic. Secondary infection of the cutaneous lesions can lead to a disfigurement of the face and hands. Porphyrins are deposited in teeth and in bones. As a result, the teeth are reddish-brown and fluoresce on exposure to long-wave ultraviolet light. (Erythrodontia)-See figure Hemolysis is probably due to the marked increase in erythrocyte porphyrins and leads to splenomegaly. Adults with a milder form of the disease also have been described.
Diagnosis
Uroporphyrin and coproporphyrin (mostly type I isomers) accumulate in the bone marrow, erythrocytes, plasma, urine, and feces. The predominant porphyrin in feces is coproporphyrin I. The diagnosis of CEP can be confirmed by demonstration of markedly deficient URO-synthase activity and/or the identification of specific mutations in the UROS gene. The disease can be detected in utero by measuring porphyrins in amniotic fluid and URO-synthase activity in cultured amniotic cells or chorionic villi, or by the detection of the family’s specific gene mutations.
Figure-2 showing Erythtrodontia in Congenital Erythropoietic porphyria
Congenital Erythropoietic Porphyria: Treatment
Severe cases often require transfusions for anemia. Chronic transfusions of sufficient blood to suppress erythropoiesis are effective in reducing porphyrin production but results in iron overload. Splenectomy may reduce hemolysis and decrease transfusion requirements. Protection from sunlight and from minor skin trauma is important. Beta Carotene may be of some value. Complicating bacterial infections should be treated promptly. Recently, bone marrow and cord blood transplantation has proven effective in several transfusion-dependent children, providing the rationale for stem-cell gene therapy.
3) Erythropoietic Protoporphyria
EPP is an inherited disorder resulting from the partial deficiency of ferrochelatase activity, the last enzyme in the heme biosynthetic pathway. EPP is the most common erythropoietic porphyria in children and, after PCT, the second most common porphyria in adults. EPP patients have ferrochelatase activities as low as 15–25% in lymphocytes and cultured fibroblasts. Protoporphyrin accumulates in bone marrow reticulocytes and then appears in plasma, taken up in the liver, and excreted in bile and feces.
Clinical Features
Skin photosensitivity, which differs from that in other porphyrias, usually begins in childhood and consists of pain, redness, and itching occurring within minutes of sunlight exposure. Photosensitivity is associated with substantial elevations in erythrocyte protoporphyrin and occurs only in patients with genotypes that result in Ferrochelatase activities below ~35% of normal. Redness, swelling, burning, and itching can develop shortly after sun exposure Symptoms may seem out of proportion to the visible skin lesions.
Although EPP is an erythropoietic porphyria, up to 20% of EPP patients may have minor abnormalities of liver function, and in about 5% of these patients, the accumulation of protoporphyrins causes chronic liver disease that can progress to liver failure and death.
Diagnosis
A substantial increase in erythrocyte protoporphyrin, which is predominantly free and not complexed with zinc, is the hallmark of this disease. Protoporphyrin levels are also variably increased in bone marrow, plasma, bile, and feces.
Erythropoietic Protoporphyria: Treatment
Avoiding sunlight exposure and wearing clothing designed to provide protection for conditions with chronic photosensitivity is essential. Oral Beta-carotene improves tolerance to sunlight in many patients. The beneficial effects of -carotene may involve quenching of singlet oxygen or free radicals.
Treatment of hepatic complications, which may be accompanied by motor neuropathy, is difficult. Cholestyramine and other porphyrin absorbents such as activated charcoal may interrupt the enterohepatic circulation of protoporphyrin and promote its fecal excretion, leading to some improvement. Splenectomy may be helpful when the disease is accompanied by hemolysis and significant splenomegaly. Plasmapheresis and intravenous hemin are sometimes beneficial.
Liver transplantation has been carried out in some EPP patients with severe liver complications and is often successful in the short term. Bone marrow transplantation is considered after the liver.