Search Your Topic
Acid-base balance-lecture 3-(role of lungs and kidney)
Normal Acid-Base Homeostasis and Role of Lungs
Systemic arterial pH is maintained between 7.35 and 7.45 by extracellular and intracellular chemical buffering together with respiratory and renal regulatory mechanisms. The control of arterial CO2 tension (paCO2) by the central nervous system and respiratory systems and the control of the plasma bicarbonate by the kidneys stabilize the arterial pH by excretion or retention of acid or alkali. The metabolic and respiratory components that regulate systemic pH are described by the Henderson-Hassel Balch equation:
pH = 6.1 + log (HCO–3/ H2 CO3)
H2 CO3 = PCO2 (mm Hg) X 0.03
Under most circumstances, CO2 production and excretion are matched, and the usual steady-state paCO2 is maintained at 40 mmHg. Under excretion of CO2 produces hypercapnia, and over excretion causes hypocapnia. Nevertheless, production and excretion are again matched at a new steady-state paCO2. Therefore, the PaCO2 is regulated primarily by neural respiratory factors and is not subject to regulation by the rate of CO2 production. Hypercapnia is usually the result of hypoventilation rather than of increased CO2 production. Increases or decreases in paCO2 represent derangements of neural respiratory control or are due to compensatory changes in response to a primary alteration in the plasma [HCO3–].
In conditions of low plasma [HCO3–] due to acidity in the medium (high H+ concentration), medullary chemoreceptors are stimulated with the resultant hyperventilation and elimination of H2CO3(CO2), the ratio of HCO–3/ H2 CO3 is restored back to normal without any net effect on pH.
The reverse occurs in conditions of high plasma bicarbonate concentration (low H +), the medullary chemoreceptors are depressed with the resultant hypoventilation and retention of CO2(H2CO3). The ratio is restored without any change in pH.
The lungs should be healthy for these compensatory changes.
III) Role of Kidney in maintaining acid-base homeostasis
Acids are added daily to the body fluids. These acids first are buffered by the HCO3 –/H2 CO3 system as follows:
H2 SO4 + 2NaHCO3 «Na2 SO4 + 2H2 CO3 «2H2O +2 CO2
The net result is buffering of a strong acid (H2 SO4) by 2 molecules of HCO3 – and production of a weak acid (H2 CO3), which minimizes the change in pH. The lungs excrete the CO2 produced, and the kidneys replace the consumed HCO3 –, to prevent progressive HCO3 – loss and metabolic acidosis, (principally by H+ secretion in the collecting duct).
To maintain normal pH, the kidneys must perform 2 physiological functions.
The first is to reabsorb all the filtered HCO3 – (any loss of HCO3 – is equal to the addition of an equimolar amount of H+), a function principally of the proximal tubule.
The second is to excrete the daily H+ load (loss of H+ is equal to the addition of an equimolar amount of HCO3 -), a function of the collecting duct.
- HCO3 – reabsorption (figure-1)
With a serum HCO3 – concentration of 24 mEq/L, the daily glomerular ultrafiltrate of 180 L, in a healthy subject, contains 4300 mEq of HCO3 –, all of which has to be reabsorbed. Approximately 90% of the filtered HCO3 – is reabsorbed in the proximal tubule, and the remainder is reabsorbed in the thick ascending limb and the medullary collecting duct.
The 3Na+ -2K+ «ATPase (sodium-potassium«adenosine triphosphatase) provides the energy for this process, which maintains a low intracellular Na+ concentration and a relative negative intracellular potential. The low Na+ concentration indirectly provides energy for the apical Na+/H+ exchanger, which transports H+ into the tubular lumen. H+ in the tubular lumen combines with filtered HCO3 – in the following reaction:
HCO3 – + H+ «H2 CO3 «H2 O + CO2
Carbonic Anhydrase (CA IV isoform) present in the brush border of the first 2 segments of the proximal tubule accelerates the dissociation of H2 CO3 into H2 O + CO2, which shifts the reaction shown above to the right and keeps the luminal concentration of H+ low. CO2 diffuses into the proximal tubular cell perhaps via the aquaporin-1 water channel, where carbonic anhydrase (CA II isoform) combines CO2 and water to form HCO3 – and H+. The HCO3 – formed intracellularly returns to the pericellular space and then to the circulation via the basolateral Na+/3HCO3 – cotransporter.
In essence, the filtered HCO3 – is converted to CO2 in the lumen, which diffuses into the proximal tubular cell and is then converted back to HCO3 – to be returned to the systemic circulation, thus reclaiming the filtered HCO3 –.
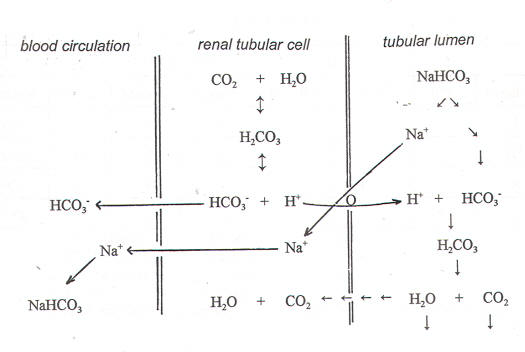
Figure-1- showing the reabsorption of HCO3-
- Acid excretion
Excretion of the daily acid load (50-100 mEq of H+) occurs principally through H+ secretion by the apical H+ «ATPase in A-type intercalated cells of the collecting duct.
HCO3 – formed intracellularly is returned to the systemic circulation via the basolateral Cl–/HCO3 – exchanger and H+ enters the tubular lumen via 1 of 2 apical proton pumps, H+ «ATPase or H+ -K+ «ATPase. The secretion of H+ in these segments is influenced by Na+ reabsorption in the adjacent principal cells of the collecting duct. Hydrogen ions secreted by the kidneys can be excreted as free ions but, at the lowest achievable urine pH of 5.0 (equal to free H+ concentration of 10 µEq/L), would require excretion of 5000-10,000 L of urine a day. Urine pH cannot be lowered much below 5.0 because the gradient against which H+ «ATPase has to pump protons (intracellular pH 7.5 to luminal pH 5) becomes too steep. A maximally acidified urine, even with a volume of 3 L, would thus contain a mere 30 µEq of free H+. Instead, more than 99.9% of the H+ load is excreted buffered by the weak bases NH3 or phosphate.
- Titratable acidity (figure-2)
The amount of secreted H+ that is buffered by filtered weak acids is called titratable acidity. Phosphate as HPO4 2- is the main buffer in this system, but other urine buffers include uric acid and creatinine.
H2 PO4 «H+ + HPO4 2-
The amount of phosphate filtered is limited and relatively fixed, and only a fraction of the secreted H+ can be buffered by HPO4 2-.

Figure-2- showing the buffering of secreted H+ by HPO4–
- Ammonia mechanism (figure-3)
A more important urine-buffering system for secreted H+ than phosphate, ammonia (NH3) buffering occurs via the following reaction:
NH3 + H+ «NH4 +
Ammonia is produced in the proximal tubule from the amino acid glutamine, and this reaction is enhanced by an acid load and by hypokalemia. Ammonia is converted to ammonium (NH4 +) by intracellular H+ and is secreted into the proximal tubular lumen by the apical Na+/H+ (NH4 +) antiporter. It can be secreted as such also and can later combine with H+ in the lumen to form NH4+
NH4 + is trapped in the lumen and excreted as the Cl salt, and every H+ ion buffered is an HCO3 – gained to the systemic circulation.
The kidneys can adjust the amount of NH3 synthesized to meet demand, making this a powerful system to buffer secreted H+ in the urine.
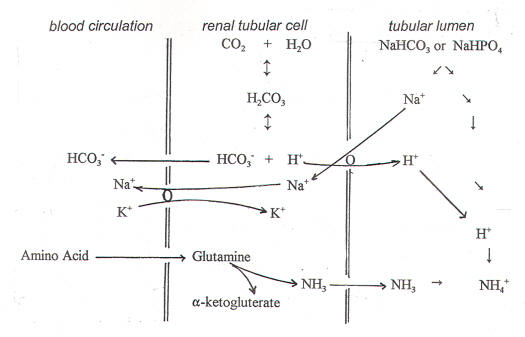
Figure-3- showing the ammonia mechanism. Glutamine is first converted to glutamate and then to alpha-ketoglutarate
